Indications
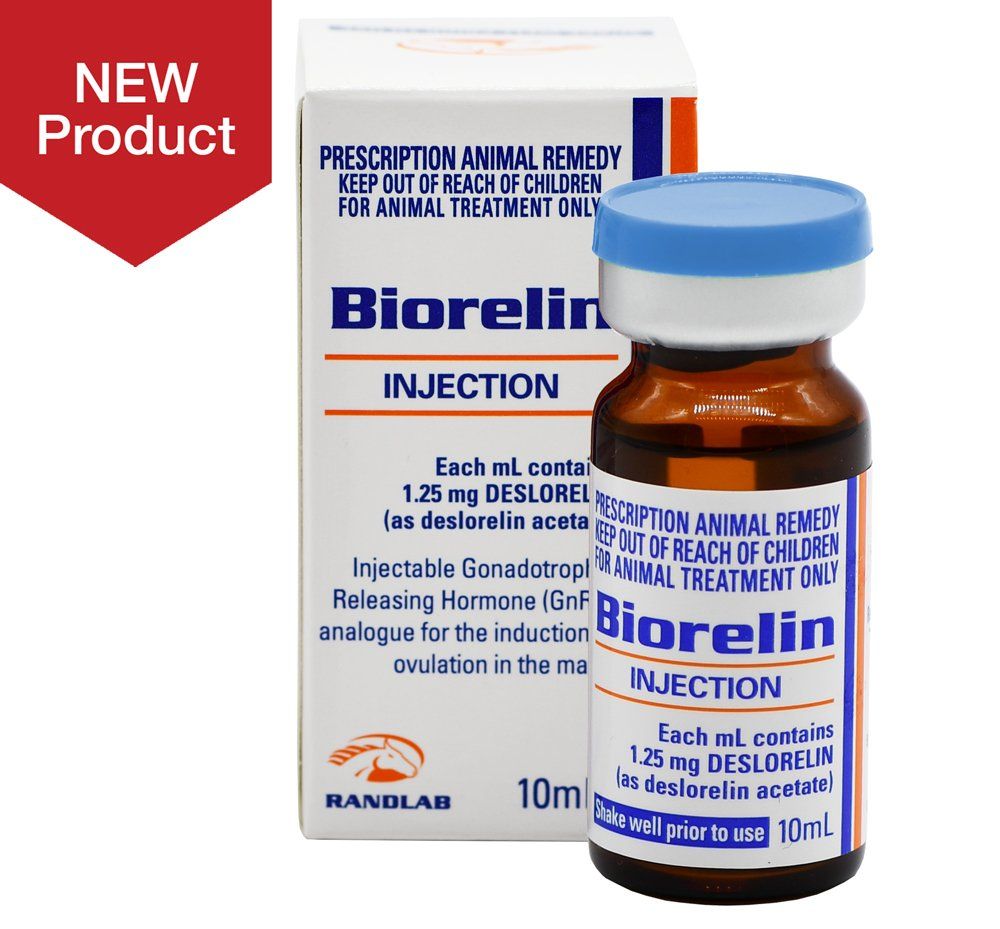
Biorelin Injection is an injectable Gonadotrophin Releasing Hormone (GnRH) analogue for the induction of ovulation in the mare.
This product is indicated for inducing ovulation within 48 hours in oestrous mares with an ovarian follicle greater than 30mm in diameter
Pharmacology
Biorelin Injection (deslorelin) is a synthetic Gonadatrophin Releasing Hormone (GnRH) for induction and timing of ovulation inoestrual mares.
The long duration of oestrus in mares, and the varying time from the onset of oestrus to ovulation, typically results in the need for multiple breedings or inseminations in order to achieve conception. Biorelin Injection induces ovulation within 48 hours after treatment in oestrous mares with a developing follicle greater than 30mm in diameter by increasing the levels of endogenous luteinizing hormone (LH).
Biorelin Injection is a sustained release, non-aqueous formulation and is indicated for the induction of ovulation in oestrous mares with an ovarian follicle greater than 30 mm in diameter.
It is intended to optimise breeding management by shortening the oestrous period, thereby allowing for stallions and assisted breeding programs to be more efficiently managed.
Following the use of Biorelin, the length of the inter-ovulatory interval between cycles is not altered.
The pregnancy rate of mares treated with Biorelin is similar to that of control mares.
Side Effects
Adverse Reactions In a clinical trial conducted in Australia injection site reactions were observed in 15.6% of treated mares. The reactions were mild to moderate swellings at the injection site which may be painful on palpation. These reactions typically resolve within 36 hours.
Dosage and Administration
For administration by intramuscular injection only. Discard vial within 28 days of initial broaching.
Shake well prior to use.
Standard Dose
Inject 1.0 ml by intramuscular (IM) injection (1.25 mg deslorelin per dose).
This product should only be administered to oestrous mares that have been demonstrated by rectal palpation and/or ultrasonography to have an ovarian follicle greater than 30 mm in diameter.
Safety Directions – Additional User Safety
Note to Handlers
Pregnant women and others of childbearing age should exercise caution when handling this product. Accidental administration may lead to a disruption of the menstrual cycle. Direct contact with the skin should therefore be avoided and any contact areas should be washed immediately with soap and water if exposure does occur. As with all injectable drugs causing profound physiological effects, routine precautions should be employed by practitioners when handling and using this product to prevent accidental injection.
Meat Withholding Period: Horses: Zero (0) days.
First Aid Instructions: If poisoning occurs, contact a doctor or Poisons Information Centre. Phone Australia 13 11 26.
Disposal: Dispose of empty container by wrapping with paper and putting in garbage.
Storage: Store below 30°C (room temperature). Store vials in an upright position. Protect from light. Do not freeze.


Reviews
There are no reviews yet.